
National recognition,
local service.
Board Certified and
Fellowship Trained Radiologists
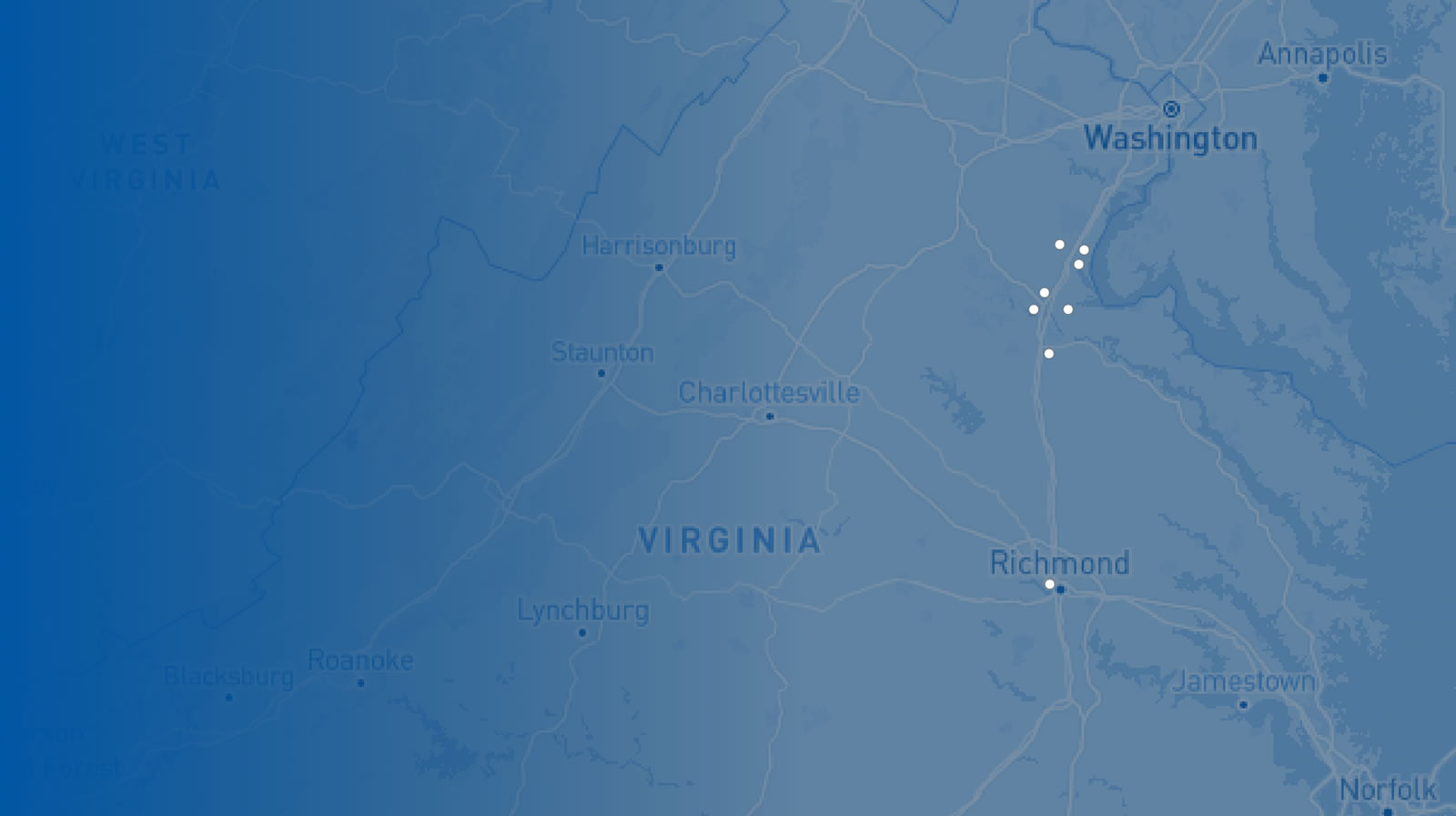
RAF Locations
- Imaging Center for Women
- Imaging Center for Women – North Stafford
- Mary Washington Hospital
- Medical Imaging - Embrey Mill
- Medical Imaging - Fredericksburg
- Medical Imaging - Harrison Crossing
- Medical Imaging - King George
- Medical Imaging - Lee's Hill
- Medical Imaging – North Stafford
- VIVA Fredericksburg
- Stafford Hospital
- Administrative Offices
Accreditations



Affiliations







